- 100% Hydrogel Device (Not a Coating)
- Nothing Sticks to It®
- Tested in Urine
- AntifoulingTM (Not Anti-Microbial)
WE FOUND ANOTHER WAY TO ENGINEER STENTS
Prolonged urological stent use is limited by biofilm formation and mineralogical encrustation. Conventional thermoplastic can range from 60 to 100% polymer, with the balance made up of radio-pacifiers and/or coatings. Alternatively, Aguamedicina® Structural Hydrogel Stents consists of less than 10% polymer, the majority of the device is water. Unlike anti-microbial coated devices which can lose effectiveness should they become damaged or suffer from erosion/diffusion, Aguamedicina® does not lose its effectiveness as the material is exposed over time to the same physiological processes. Aguamedicina® does not require the anti-microbial additives typically found in coatings of a conventional “anti-microbial” material to diminish biofilm build-up. This performance, Antifouling®, is an inherent characteristic of Aguamedicina® primarily due to the high aqueous content of the material.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
A rEVOlUTIONAry IDEA WHErE THE COATINg IS THE STENT
The Q Urological Corporation’s Aguamedicina® Hydrogel is a compliant and stent bio-material that absorbs the physiological and peristaltic energies associated with and generally responsible for stent migration. Due to its novel design and material, the stent will act like a spring or shock absorber.
DEVElOPED WITH PErfOrMANCE IN MIND…
Biocompatibility
The Polyurethane sample at 10 X magnification of surface shows typical encrustation.

DEVElOPED WITH PErfOrMANCE IN MIND…
Aguamedicina
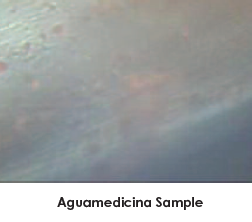
The Aguamedicina® sample at 10 X magnification of surface shows no sign of encrustation.